Research in the Mehrkhodavandi group focuses on catalysis. We seek to discover molecular structure and reactivity that can contribute to interdisciplinary solutions for environmental challenges in both fundamental and applied studies. The lab focuses on rational catalyst design using synthetic inorganic and organometallic chemistry, polymer synthesis, and extensive mechanistic studies. We place a great emphasis on the applicability of our research and have active collaborations with industry partners to develop biodegradable polymers of interest in medicine and materials.
Selected Recent Publications:
-
“Selective Block Copolymerization of Lactide and Methyl Methacrylate with Cationic Indium Catalysts: Exploring the Influence of Noncovalent Ligand Interactions” ACS Catal. 2025, 15, 9, 7588–7600
-
“Synthesis of High-Molecular-Weight Poly(ether-alt-ester) by Selective Double Ring-Opening Polymerization of Spiroorthoesters” ACS Macro Lett. 2024, 13, 2, 266–272
-
“Alkyl initiated ring opening polymerization of lactide by indium complexes supported by NCN pincer ligands”
Dalton Trans., 2024, 53, 19337–19341 -
“Mechanistic Insights into Selective Indium-Catalyzed Coupling of Epoxides and Lactones”
ACS Catal. 2023, 13, 20, 13195–13204 -
“Impact of counterion valency on the rheology of sulfonated cellulose nanocrystal hydrogels”
Carbohydr. Polym., 2023, 302, 120378
Current Researches
Joseph’s Work
Polymers containing sulfur and sulfur-containing functional groups have numerous beneficial properties in applications ranging from self-healing materials to antibacterial surfaces. Joseph aims to utilize functional-group tolerant indium catalysts to introduce sulfur motifs into well-known polyesters such as poly(ε-caprolactone) and poly(lactide) to tune their degradation characteristics and/or physical properties, all while exploring the behavior of sulfur-containing monomers in indium-based catalysis.

Jason’s work
Developing bio-derived materials are essential to replace petroleum-based polymers for their sustainability. Jason’s research integrates catalysis, polymer science, and materials engineering to develop bio-based non-isocyanate polyurethanes (NIPUs). By valorizing renewable feedstocks like lignin, vegetable oil and CO2, he designs synthetic routes to create NIPUs with tunable mechanical properties and recyclability. His work aims to create a sustainable life cycle for next-generation polymers, transforming waste streams into value-added sustainable materials.

Kritika’s work
Synthesizing amphiphilic block copolymers with controlled molecular weight and dispersity is crucial for achieving desired morphologies upon self-assembly. Kritika’s work focuses on developing biodegradable amphiphilic block copolymers for biomedical applications. By synthesizing and functionalizing these polymers with well-controlled molecular weight and dispersity, she is able to guide their self-assembly into specific nanomorphologies. These functionalized polymers are further utilized for targeted delivery and diagnostic applications.

Takeo’s work
Takeo’s research aims to synthesize low valent group 13 metal complexes and explore their role in small molecule activation. Their highly reactive nature, along with their dual Lewis acidic and Lewis basic character makes these complexes interesting to study. He is interested in synthesizing these low-valent complexes through mechanochemical methods and exploring the mechanisms for the oxidative addition to the low-valent complexes. The synthesis of aluminyl and indyl complexes is also of interest for the C-H activation of otherwise inert substrates.

Justin’s work
Compared to their linear counter parts, cyclic polymers exhibit interesting and unique properties such as increased crystallinity and lower intrinsic viscosity which could lead to novel material properties. However, their material properties remain under-explored due to the difficulty in synthesizing them at high molecular weights. Justin’s research looks to understand and develop catalyst systems for the synthesis of biodegradable high molecular weight cyclic polymers, with the goal of increasing their ease of synthesis. This would ultimately allow exploration of their material properties and expand their application.
Jae’s work
Block copolymers are valuable materials because their differing blocks allow for tunable properties, making them useful in biodegradable plastics and medical applications. Jae’s research focuses on using cationic indium complexes to catalyze the synthesis of a variety of block copolymers made of methacrylate and cyclic ester monomers. These materials combine strength and flexibility with biodegradability, and through improving catalyst design, she hopes to explore the functional tolerance of indium catalysts.
Dinuclear catalysts for controlled ring opening polymerization of cyclic esters
The interests of the group encompass the areas of synthetic and catalytic organometallics chemistry, with a special focus on control of chirality and electronics via rational ligand design. In one major part of the project, we are developing Lewis acidic metal centres supported by chiral ligands for the controlled ring opening polymerization of cyclic lactones to form biodegradable polyesters.

Fig 1. Ring opening polymerization of lactide catalyzed by a dinuclear catalyst. Both metal centres are involved in propagation.
Highly active and enantioselective catalysts
We reported the first chiral indium salen catalyst that displays a remarkable combination of high activity and isoselectivity for the polymerization of racemic lactide. This is in contrast to chiral aluminum salen complexes, which are more selective yet suffer from low activity. Based on preliminary kinetics studies, this system shows a high degree of enantiomorphic site control derived from ligand chirality, resulting in the formation of stereoblock PLLA-b-PDLA with significant crystallinity.
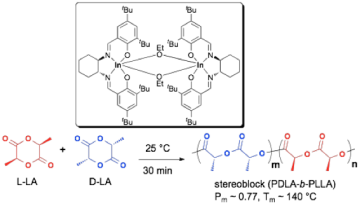
Fig 2. Chiral indium salen complexes are highly active, isoselective catalysts for the ring opening polymerization of racemic lactide. Preliminary kinetic investigations confirm enantiomorphic site control as the dominant contributor to selectivity and formation of block copolymers.
Immortal ring opening polymerization of cyclic esters
The ethoxy-bridged dinuclear indium catalyst can used for the ring opening polymerization of the cyclic ester beta-butyrolactone (BBL) to form the biodegradable polyester poly(hydroxybutyrate) (PHB). The catalyst shows remarkable activity and control during polymerization, allowing for formation of diblock polymers. Addition of high ratios of alcohols to the catalyst leads to fast chain transfer and immortal polymerization of lactide as well as BBL.
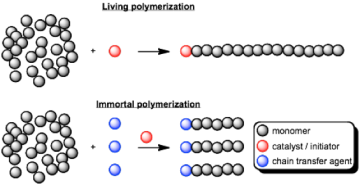
Fig 3. Living vs. immortal polymerization. the Chain transfer agent can be another polymer such as poly(ethylene glycol), leading to biodegradable amphiphilic diblock polymers.